Overview
Research in the Brewster lab aims to understand fundamental aspects of brain development, oxygen homeostasis and metabolism, using the genetically tractable zebrafish embryo as a model system. We use a combination of high resolution and time-lapse microscopy, genetics, cell and molecular biology to investigate these general questions:
Brain development
- What are the dynamic processes that shape the neural tube (the precursor of the brain and spinal cord)?
- Which signaling molecules regulate brain development?
- Are mutations in genes encoding these signaling molecules genetic risk factors for human brain birth defects?
Oxygen homeostasis and brain metabolism
- How do cells sense low oxygen and respond in an adaptive manner?
- Are hypoxia-responsive genes in organisms that have high tolerance to low oxygen (such as the zebrafish embryo) also active in human cells?
- If so, can modulation of the activity of these genes prevent hypoxic injury in human organs and tissues?
Brain Development
A long-term research interest of the Brewster lab is to understand the cellular and molecular mechanisms that drive the formation of the neural tube (the precursor of the brain and spinal cord), a process otherwise known as neurulation. Neural tube defects (NTDs) are the second most common structural birth defect (1 of every 1000 births), making it a major public health burden that has lifelong implications for affected individuals, including paralysis, bowel and bladder dysfunction and cognitive difficulties. Despite the high frequency of NTDs, much remains to be learned about their causes and possible treatments.
Understanding the Mechanics of Neurulation
A challenge in understanding the morphogenetic movements that shape the mammalian neural tube is that they occur very early in development when access to the embryo is limited. The zebrafish model circumvents this problem as embryos are born and develop externally – attach movie of developing zebrafish embryo (bright field).
Furthermore, zebrafish embryos are transparent, which facilitates the imaging process. It is therefore possible to capture the dynamic process of neurulation in zebrafish
embryos via time-lapse imaging and high-resolution microscopy (Figure 1 and Movie 1).
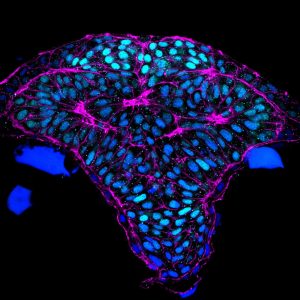
Fig 1. Cross section through the developing zebrafish forebrain. Light blue: telencephalon, magenta: cortical phalloidin.
Movie 1. Optical cross section through the developing foregrain. Medial hingepoint cells are labeled in magenta.
Previous Work. By developing tools for imaging cells in the brain at high resolution, the Brewster lab established one of the first dynamic models for how neural tube development is orchestrated in the zebrafish embryo. These studies provided a framework for exploring the molecular pathways that shape the neural tube and revealed that neurulation in zebrafish presents many similarities to this morphogenetic process in mammals but also some unique features (Werner et al. 2021). Interestingly, our most recent study revealed the presence of neural folds and hingepoints in the zebrafish developing forebrain, which are classic features of mammalian primary neurulation. The significance of this finding is that the zebrafish is a relevant model to understand normal and abnormal aspects of human brain development.
Ongoing Work. The ability to readily image the dynamics of neurulation in zebrafish has allowed us to not only reveal evolutionary conservation of this process in zebrafish, but also to discover novel aspects of neurulation, which are likely to be relevant to humans. We have recently identified the presence of “reverse hingepoints” (RHP) in the zebrafish forebrain, which we believe to be essential for elevating the neural folds. If confirmed, we can begin to unravel the molecular mechanisms that regulate RHP formation, which may lead to the identification of novel genetic factors that contribute to human NTDs.
Identifying the Molecular Pathways that Regulate Neurulation
In addition to understanding the dynamic cell shape changes and behaviors that shape the neural tube, we are also interested in revealing the genetic pathways that regulate them – as these genes are predicted to be essential for brain development and mutations in them may represent novel genetic risk factors for NTDs . Over the past decade, we have identified multiple signaling molecules that drive neural tube formation in zebrafish. In particular we have uncovered a previously unknown role for the microtubule cytoskeleton and several microtubule regulators – Pard3 (a regulator of cell polarity), MAP1B (a microtubule associated protein), and the ligand/receptor pair Repulsive Guidance Molecule (RGM)a,b/Neogenin (Brown et al. 2019) in mediating cell elongation and the establishment of cell polarity, two cell behaviors that are essential for proper neural tube formation.
Future Directions
Epidemiology studies have revealed that folic acid, a vitamin B derivative, significantly reduces the risk of NTDs in humans, demonstrating that interventions can be successful. However, 30–50 % of NTDs are not folic acid-responsive. It is estimated that up to 70% of the risk associated with NTDs is attributable to genetic factors but their identity remains for the most part unknown. With the advent of next generation sequencing, there has been an exponential increase in the number of genetic variants identified in NTD patient cohorts. Demonstrating causality of these variants and their possible genetic interactions represents a critical bottleneck in the field for identifying potential new interventions and informing genetic counseling. In the future, we propose to use the zebrafish as a model to functionally characterize human NTD variants.
Oxygen homeostasis and brain metabolism
The brain and other metabolically-demanding organs are exquisitely sensitive to fluctuations in oxygen levels, as oxygen is essentially to generate cellular energy in the form of ATP. Oxygen deprivation (hypoxia or anoxia) occurs frequently as a consequence of premature birth, disease (e.g congenital heart disease, stroke, vascular occlusion, asthma, sleep apnea, etc), accident (choking, suffocation, head trauma, etc) or even under normal circumstances such as life at high altitude. Lack of oxygen can trigger cell death in the brain causing cognitive and motor malfunction or even death of the organism – with stroke ranking as the third leading cause of death in the US. In contrast to
the sensitivity of normal cells to oxygen deprivation, cancer cells in the core of solid tumors are resistant to low oxygen. This remarkable anoxia tolerance is also observed in several vertebrates, including zebrafish, that are known to survive oxygen deprivation for prolonged periods (ranging from several hours to a couple months). The mechanisms underlying anoxia tolerance are mostly unknown, however, it is thought that metabolic suppression and arrest of cellular activity (proliferation, migration, …) are key events (Fig 2).
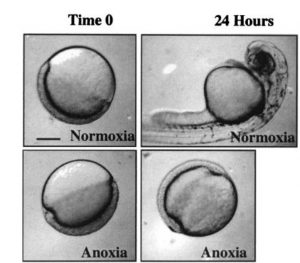
Figure 2. Zebrafish arrest development in response to oxygen deprivation. Image obtained from Padilla and Roth, 2001.
A relatively new line of research in the Brewster lab aims to characterize the response to loss of – and re-introduction of oxygen in the hypoxia-tolerant zebrafish embryo. We have identified a family of molecules encoded by N-myc Downstream Regulated Genes (NDRGs), that play an essential role in the physiological adaptation of zebrafish brain cells and cells in other metabolically-demanding organs to low oxygen. We are interested in understanding how these genes are regulated at the transcriptional (Le et al., 2020 PREPRINT) and protein level by low oxygen and how they function to prevent hypoxic injury. Since humans are highly sensitive to fluctuations in oxygen, we are further interested in knowing whether NDRGs are differentially regulated in human cells and whether modulation of their activity can mitigate hypoxic injury.